Tavanta’s product TAVT-119 is currently in development for anal fissure, a painful colorectal condition with a significant unmet medical need.
Overview of Anal Fissure
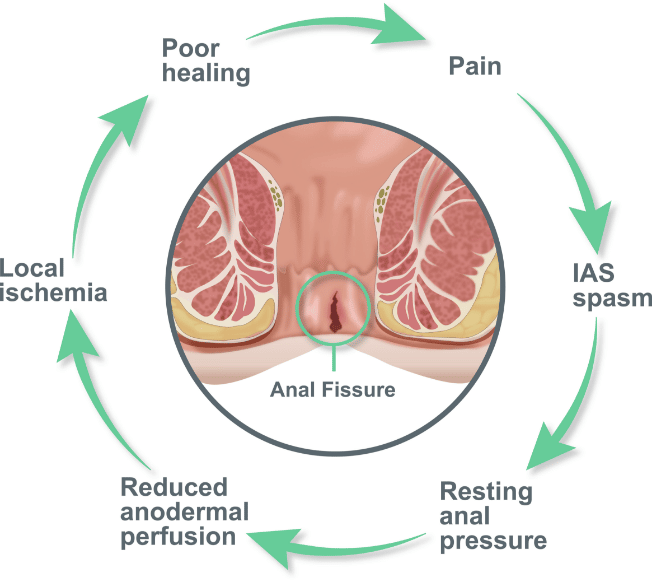
An anal fissure is a longitudinal tear in the skin of the anal canal, causing significant pain and debilitation, and is commonly associated with constipation. The primary symptom of anal fissure is severe pain (including intense spasms) with or without superficial bleeding during and after defecation.1,2 The condition interferes with physical activities, work, social life, and psychological well-being.
More than 500,000 diagnoses of anal fissure are made each year in the U.S. However the true prevalence of anal fissure could be much higher since patients are frequently misdiagnosed.
Current Treatments and Unmet Needs
For anal fissure, topical nitrates (including Rectiv®), or calcium channel blockers (typically diltiazem or nifedipine) are recommended as first-line treatment1,2, and are aimed at interrupting the cycle of anal spasm, reduced blood flow and pain.
The use of Rectiv (nitroglycerin) Ointment 0.4% is limited by its poor tolerability (primarily headaches that lead to early discontinuation), drug-drug interactions, and uncomfortable intra-anal administration.3,4
There are no FDA-approved calcium channel blockers for the treatment of anal fissure in the US, with these agents only available through limited compounding pharmacies on an off-label basis. Compounded drugs have several drawbacks, including variability in final product quality, and they are often not reimbursed.5,6
TAVT-119
TAVT-119 is an amlodipine besylate gel in development for pain associated with anal fissure. Amlodipine is a long-acting calcium channel blocker that can relax smooth muscles and improve vasodilation.7
TAVT-119 is designed to reduce anal fissure pain while avoiding some of the limitations associated with compounded agents. We are evaluating the safety and efficacy of TAVT-119 in the clinic as a treatment for moderate to severe pain associated with anal fissure.
If successful, TAVT-119 will be the first FDA-approved calcium channel blocker for anal fissure.
1 Davids JS, Hawkins AT, Bhama, AR, et al. The American Society of Colon and Rectal Surgeons Clinical Practice Guidelines for the Management of Anal Fissures. Diseases of the Colon & Rectum. 2023; 66(2), 190–199.
2 Wald A, Bharucha AE, Limketkai B, et al. ACG Clinical Guidelines: Management of Benign Anorectal Disorders. Am J Gastroenterol. 2021;116(10):1987-2008.
3 RECTIV® (nitroglycerin) Ointment 0.4% – Prescribing Information: https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/021359s000lbl.pdf
4 Hyman NH, Cataldo PA. Nitroglycerin ointment for anal fissures: effective treatment or just a headache? Dis Colon Rectum. 1999;42(3):383-385.
5 Sellers S, Utian WH. Pharmacy compounding primer for physicians: prescriber beware. Drugs. 2012;72(16):2043-2050.
6 Shah M, Sandler L, Rai V, Sharma C, Raghavan L. Quality of compounded topical 2% diltiazem hydrochloride formulations for anal fissure. World J Gastroenterol. 2013;19(34):5645-50.
7 NORVASC® (amlodipine besylate) Tablets – Prescribing Information: https://labeling.pfizer.com/showlabeling.aspx?id=562